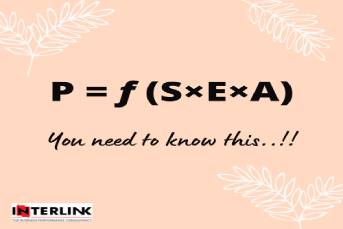As the healthcare scenario is experiencing the paradigm shift from cure to care, preventive health has received generous importance and hence the biologics. Growing demand for vaccines, monoclonal antibodies and biosimilars are creating excellent business space for Indian pharma.
Indian pharmaceutical sector has great potential to earn the identity of global hub for manufacturing biologics. The ability to invent globally competitive, affordable and novel vaccines and biosimilars is emerging as one of the greatest driving forces for Indian pharma. As the industry is shifting from chemical-based drugs to biosimilars and biologics, this scenario presents excellent opportunities for Indian pharma industry in the space of life sciences and biotechnology.
Till September 2019, India received over 98 biosimilars approvals in the domestic market, more than any other country. Moreover, the approvals which we are receiving for biosimilars in regulated market, further boosting the confidence and willingness of Indian pharma players to contribute in global market shares. Owing to the rising domestic demand, potential and investments in biologics in India, according to the reports, more than 40 biosimilars reached clinical development stage in India which is far more than that of United states and similar to European Economic Areas.
Following elements will ensure India’s march towards achieving the title of ‘fastest-growing bio hub’-
Vaccines establishing strong presence
Fulfilling over 60% of global vaccine requirements, India is now the largest volume supplier of vaccines to public market. This is the outcome of ongoing critical focus on R&D and mass manufacturing. Moreover, companies in India increasingly investing in innovative approaches toward vaccines for common diseases further making the space lucrative. Some of the examples of this innovative approaches include typhoid conjugate vaccine, “eco-friendly” recombinant Hepatitis-B vaccine (free of cesium chloride and thiomersal) and Serum Institute’s liquid rotavirus vaccine of Bharat Biotech. Such approaches target different aspects like improving compliance, improving stability by adjusting formulations and several other fundamentals to upgrade and enhance existing products.
Moreover, government is also boosting India’s presence in vaccine market through funding and investing in R&D. Department of Biotechnology (DBT), the Ministry of Health and Family Welfare (MoHFW) and the Indian Council of Medical Research (ICMR) are playing wisely to propel the vaccine market.
Market players are driving biologics
Indian market players are all set to propel the growth of biologics and biosimilars in India and globally. Their efforts are paying off and positive outcomes are seen in terms of revenue generation and overall reputation in the international markets. Domestic biosimilars market generated over US$576 million in 2019 while achieving the growth rate of about 11% (2018 revenue- US$520 million).
By developing novel monoclonal antibodies (BIOMab EGFR for head and neck cancer treatment in 2006 and Alzumab (itolizumab) for psoriasis treatment in 2013), Biocon became very first company to launch indigenously developed novel biologics in India. In collaboration with global companies, these antibodies have been launched in many other countries which has greatly influenced Indian pharma industry to dive deeper in the world of biologics. The company aims to generate around INR7,460 crore by 2022 alone from biosimilars business. Looking at the revenue of INR1,951 crores in 2019-20, the target looks genuine too. Moreover, the company in collaboration with Mylan has entered in US market with their first biosimilars for Herceptin and Neulasta, which further opening the revenue streams for the nation.
Zydus Candila, who is exploring the use of long-acting interferon alpha-2b for treating Covid-19, can also generate significant opportunities for Indian biologics in international markets. The biosimilar version of this immunomodulator is already being commercially manufactured by the company for the treatment of Hepatitis B and C.
Government priority- bio-tech based drug development
Under ‘Make in India’ campaign, Indian government is actively promoting the biotech-based drug development in the country through various approaches including effective fundings and investments. Initiatives introduced by DBT and the Biotechnology Industry Research Assistance Council (BIRAC) are favourable enough to transform India into biotechnology-based innovation and research hub. Such initiatives are policy making, promoting industry-institute partnership, generating entrepreneurship cells, etc. Moreover, Public Private Partnerships are also motivated in order to captivate investments and fundings from investors, industry and other agencies.
Securing future with Biologics
Being the largest provider of generics globally, Indian is now looking for expansion beyond generics while exploring opportunities in biologics and biosimilars. More than 10 blockbuster biologics (with total revenue of USD 60bn) are losing their patents in next 2-3 years, creating amazing revenue stream for India through biotech sector. Moreover, first-time ANDA approval from USFDA makes the scenario even more favorable for Indian pharma.
We have over 200 biosimilars in pipeline with the collective contribution from more than 52 Indian companies. However, the number of companies penetrating the US and European market is significantly low despite of the largest number of approved biosimilars in India. One of the restraining factors could be nonalignment of India’s regulatory guidelines with these markets. More efforts are necessary in the regulatory space.
Moreover, strengthening testing requirements for biosimilars, improving animal testing and increasing the number of patients in clinical trials are some of the elements of development which need attention and efforts to emerge as a ‘fastest-growing bio hub’ in the future.
The article is written by Dr. Smarta and is published by Healthcare India Today