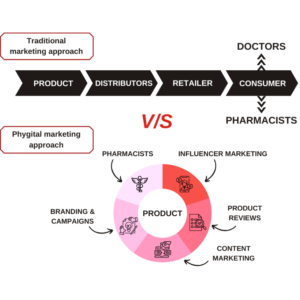
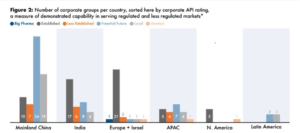
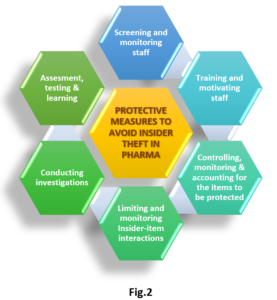
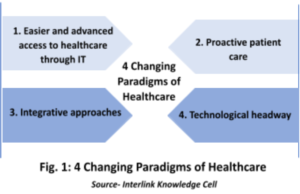
Generic drug market, one of the positively impacted markets during pandemic had witnessed over $ 4911.6 billion of revenue in 2020 and expected to account for about $650.3 billion by 2025 with the CAGR of 9.6%.
One of the greatest reasons behind this astonishing growth would be increased healthcare expenditure during the pandemic and collapsing economies. Due to rising unemployment during the period of shutdown, governments of various nations employed several strategic initiatives in order to fulfil the ever-growing demand of cheaper substituents of pharma drug. And one of those initiatives was ensuring the availability of low-cost generics to the population.
Moreover, factors like patent expiration of blockbuster drugs, increasing incidences of lifestyle diseases including acute and chronic conditions and rise in geriatric population are also positively propelling the generics market.
Why generics were born?
Let’s have a look at 2As behind the origin of generic drugs-
Affordability
The company who holds the patent for particular drug (brand drug), applies its own pricing policy which make these drugs expensive and unaffordable for most of the population. On the other hand, for the generic drug, an off-patented brand drug, whose costings are decided through government interventions, are very affordable. This affordability is influenced by various aspects such as, companies making generic drugs need not repeat lengthy and costly research and development in laboratories as it is available readymade through patent-holding company.
As generics were developed, people who were losing on crucial medications due to their higher prices, were able to afford the essential treatment for them and their loved ones without pouring all their life savings.
Life-threatening health condition such as blood cancer, due to which many lives were sacrificed owing to non-affordability of branded medicine costing Rs. 1,14,400 per month, whose generic version is now available at as low as Rs. 11,400 per month. Same repeats for major heart disorders and other chronic illnesses.
As per World Health Organization (WHO), in developed and developing countries, if the prescription of generics is increased by the medical professionals, the health expenditure can be reduced by huge 70 percent.
Accessibility
Manufacturers holding patents for expensive brand drugs, used to enjoy the patent exclusivity for too long until FDA started taking serious efforts to prohibit extended ownership of such patent. To enhance the accessibility of such branded drugs to developing countries, who can’t afford the cost of research and development, this step of FDA was essential.
Owing to this, essential drugs were available for various levels of society including rural areas. Chemists in rural areas never used to serve branded drugs due to high prices so the population had no enough access to crucial drugs. As alternative options like generics are more affordable, they are much accessible too for different groups of society belonging to different financial levels.
Moreover, several generics give rise to price competition due to which patients are accessible to better treatment alternatives at same or lower prices.
FDA’s routine evaluation of generic drug programs is resulting into increased funding for generics production, staff recruitment and other factors which making the generics scenario much approachable.
For developing countries like India, this two factors, affordability and accessibility, were very crucial as back in 1960s, most of the Indians were not holding health insurance and the salary structure was too weak as compared to the western countries. Domestic production of low-cost generics was mandatory for India owing to such factors.
Indian pharma was doing well in pharma market owing to its capabilities to produce cheaper medicines. In 2005. Indian signed an agreement with World Trade organization which significantly changed the pharma scenario in India and opened several opportunities. That agreement was none other than- Trade-Related Aspects of Intellectual Property Rights (TRIPS).
Today, India is known as world’s largest provider of generics. As the medicines produced in India are 60% cheaper than the medicines produced in US, many western countries are outsourcing their production of medicines to India which nourishing the increasing globalization of Indian pharma industry.
Future of generics
Innovations are going to rule the entire generics space in the coming years, innovators and manufactures will test their unique perspective toward this market to enjoy highest possible revenues. Discussed ahead are some future-oriented approaches which can be seen in generics field.
Traceability mechanisms will intensify
Traceability will be one of the most crucial systems which need to be employed, compulsorily, by pharma companies in the coming years. When it comes to generics, counterfeit drugs are the issue of concern. For traceability of mainstream supplies containing fake drugs requires high-end technology like blockchain architecture which can be seen much prevalent in the future scenario.
There are various architectures of blockchain which pharma industry will adopt in coming years in order to provide lifesaving information on the formulations. In addition to counterfeit alert, conformation status along with expiry date, non-conformities, etc., efficient waste management can also be achieved with improved workflow.
Not only volume to value shift in the product can be seen, but quality of delivery will also be seen enhanced in the coming years.
Complex generics and NDDSs will take a leap
In competitive generics market, Novel Drug Delivery Systems (NDDSs) and complex generics are major differentiating factors dominating the pharma market. For country like India, NDDS-based generics development is quite economical and less insecure. So, the industry can explore novel drug delivery-oriented approaches to serve upgraded generics in the future.
Moreover, complex generics, which are often employed for cancer like challenging diseases can be developed to the greater extent as the companies are adapting more expertise, sophisticated groundwork and development strategies required for complex generics.
Use of Artificial Intelligence (AI) and 3D printing will skyrocket
AI in current scenario tackling many challenges related to data management, in-house R&D, studying and repurposing old (or failed) drugs, etc., generic drug producers will extract highest possible advantages through AI related technologies.
AI is the future of pharma industry which will save generous amount of money and time required for complex processes of generic drug development and marketing.
3D printing is one of the major technological revolution through which production of novel medicines and devices is way too easier as time investment required is lesser and the outcomes are amazing. When FDA approved first commercial 3D tablet called Spiratam (levetiracetam), 3D printing technology gained tremendous attention and it is poised to become a highly employed technology in pharma industry.
Bio generics will bloom
Although the generic biopharmaceuticals are not easily approved by regulatory systems owing to the complications associate with the replication of complex manufacturing processes of biopharmaceuticals, producers nowadays are exploring several ways to catch the norms essential to launch bio generics.
Drug producers will invest in large scale clinical programmes in order to generate efficacy and safety evidences for their bio generic drugs. Various efforts can be made to produce highly controlled quality system, manufacturing and even transportation and storage for bio generics to withstand stringent quality standards of biopharmaceutics.
Generic companies will have to test some alternative and advanced strategies to scale-up in the coming years. Volume to value shift is necessary through innovative technological interventions. Digital technologies nowadays are effectively lowering the cost of healthcare and uplifting the high-quality generic production which is a good way to sustain in future.
Besides this, number of patents are highly complex which can generate the risk of litigation as many a times products, devices, manufacturing processes are also patented. This issue has to be handled wisely in order to prevent the loss of money and efforts.
The future of generics is going to be full of innovations accompanied with sophisticated technologies in each and every process ranging from research and development, quality production and delivery to marketing of the product. Quality and safety will acquire immense attention and improved delivery will be a major game. Producers will find new ways to tackle current challenges associated with biosimilars and bio generics like complex formulations. With this, let’s hope that the future of healthcare will be much more affordable and accessible than ever.