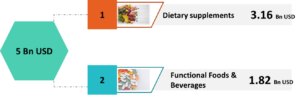
Healthcare systems are undergoing a paradigm shift, especially in this age of innovation. The focus today is on care rather than cure thus proving the age old proverb “prevention is better than cure” to be right. “Under the present situation, people are gaining trust on herbal medicines. Owing to changing trends among people, who want to be healthy and not to fall sick, they are opting for preventive options. This shift from cure to care has been one of the crucial reasons for the acceptance of herbal drugs including phytodrugs, a purified bio-active fraction made from the extract of medicinal plant or its part,” Dr. R.B. Smarta, Founder and Managing Director of Interlink, a business performance consulting firm operating in the pharmaceuticals, phytopharmaceuticals, nutraceutical and wellness space for over 35 years, says in an exclusive e-mail interview with Trinity Mirror. Dr. Smarta shares his views on phytopharmaceuticals and the emerging opportunities in the Indian market in a free-wheeling interview. Excerpts:
In what ways are phytopharmaceutical medicines better than the traditional drugs?
Even though, both traditional medicines and phytopharmaceuticals are derived from plant sources (and other natural sources except for phytomedicines), in India, regulatory bodies controlling these two medicinal practices differ significantly. ASU (Ayurvedic, Siddha and Unani) medicines are regulated under the purview of Department of AYUSH, while, phytopharmaceuticals are regulated by CDSCO (Central Drugs Standard Control Organisation). Owing to this, phytopharmaceuticals are controlled in the similar manner as conventional chemical drugs. Their safety, quality control and efficacy are proved and supported by randomised, double-blind, placebo-controlled clinical trials which result in evidence-based drug development. This scientific evaluation of phytopharmaceuticals makes them more admissible than traditional medicines which are often supported only by non-clinical data which is not so reliable. Moreover, to develop phytodrugs, only therapeutic actives are isolated from plant sources through specialised extraction techniques so that the highest therapeutics efficacy of the particular bioactive can be employed for a specific disease.
Can you share your journey on the drugs and phytopharmaceuticals domain?
I have been in the pharma industry for the past 50 years and in the nutra, plant-based products, phytopharmaceutical industry for the last 20 years. Interlink being a consultancy firm, along with my team, we are provide go-to market strategies, launch new companies with a focus on top line products and profitability. This is the journey that has been going on so far.
Medical fields like allopathy promise cure for over 85 per cent of the current day diseases. Can you assure an equal or better cure?
As phytopharmaceutical field is still at a formative stage in India, it can’t be entirely compared to allopathic medicines which have established an irreplaceable place in healthcare practices after decades of research and development. True efficacy and the frequency of side effects of herbal medicines are not known as very few have undergone large clinical trials such as allopathic drugs. Although, till today, very few herbs are scientifically evaluated and have the potential to be used in medical treatments, extensive research in this area can bring out the true potential of phytopharmaceutical market in the near future.
What are the milestones crossed by phytopharmaceuticals in terms of human health?
Traditional herbal medicines in India and even Chinese traditional medicines have emerged as an excellent source of phytopharmaceutical bioactive, which is extensively being studied and utilised today.
Most of the people are comfortable with the current allopathy treatments and medicines. Do you think that phytopharmaceutical drugs earn people’s trust?
Yes, they can and the pandemic has led a rigid platform for this trust in plant-based medicines. The changing perspective of patients towards chemical drugs due to increasing incidences of side effects and addiction is further propelling the acceptance of herbal medicines. The initiatives by the Government of India and Ministry of AYUSH during COVID have promoted herbal medicines to a very huge extent, people are gaining trust in herbal medicines, which is a great sign for phytodrugs as well. Owing to the changing trends among people today, they don’t want to fall sick and want to remain healthy using possible preventive measures, there is a paradigm shift from cure to care. This has been one of the crucial reasons behind the acceptance of herbal drugs. Allopathic drugs can’t be replaced entirely from the regimen but looking at the increasing prescription of herbal medicines from physicians, use of synthetic drugs for longer time period can be prevented and further treatment can be followed by herbal medicines for the long-term to avoid addiction and risk associated with allopathic drugs.
Where do phytopharmaceuticals stand in cancer treatment?
Naturally occurring phytochemicals such as vinca alkaloids (vincristine, vinblastine, podophyllotoxin), taxol analogs have been extensively studied for the cancer therapy. Such bioactives proved to be responsible for inhibiting growth and progression of cancer through mechanisms such as proliferation inhibition, carcinogen inactivation, cell cycle arrest induction, increasing antioxidant and apoptosis. In current cancer therapies with phytochemical drugs, four major classes of anticancer compounds namely, vinca alkaloids, camptothecin derivatives, epipodophyllotoxin, and taxane diterpenoids are employed. Additionally, other plant-derived anticancer agents are also being used and some of them are under investigation. Although, several pre-clinical and clinical trials give satisfactory evidences about such anticancer phytochemicals, some challenges such as molecular interaction with different ligand molecules, different mechanism of action,etc need to be validated with further research and large scale in-vitro/in-vivo studies to full proof the therapy.
How useful are phytopharmaceutical drugs in treating COVID? Are there any studies conducted to prove the efficacy of the drugs on COVID-affected?
Several phytochemicals have proved to be efficacious against various viral infections. Chinese traditional medicines is a well-established practice in this area. In India, the very first phytopharmaceutical drug for the treatment of COVID-19, called AQCH (derived from the tropical shrub Cocculus hirsutus), received approval from the Drugs Controller General of India for clinical trials. However, it did not receive grant for ‘Emergency authorization status’ from government. Various efforts are still going on in this space to develop a promising plant-based treatment for COVID-19. Moreover, herbs like Glycyrrhiza glabra, Withania somnifera, Timospora cordifolia and AYUSH-64 (Polyherbal AYUSH drugs) have been taken up for clinical trials against SARS-CoV2 virus by Ministry of AYUSH and CSIR.
When it comes to branding of traditional medicines, people tend to develop a reluctance. What do you think is the reason for this behaviour? How can this be rectified?
This is because of the huge difference between the marketing approval process and criteria in India and abroad for traditional herbal medicines. India is facing challenges in terms of regulations due to which uncontrolled and substandard formulations along with misbranding is an uncontrollable scenario. India is exporting herbal ingredients but the speed and quantum is relatively less. Moreover, production of fraudulent herbal medicines, which are deficient in clinical data are very common in India ,and is emerging as a roadblock for branding and marketing of such products. We need a modern regulatory system all the way from cultivation and export of herbal medicines in order to develop a huge source of income. Quality control, standardisation, clinical trials, marketing strategies require certain policies through Government interventions to meet international standards of selling plant-based products. Additionally, checking therapeutic claims is one of the crucial steps that needs to be followed vigilantly.
Why does research take a back seat when it comes to traditional medicine in India?
Variable and inconsistent results along with inadequate research designs for herbals is one of the major limitations while promoting plant-based products. Studying plant-derived extract is pretty complicated owing to the presence of cluster of complex chemical compounds in a single herb. Such research and development require highly specialised techniques, devices and technologies which are deficient in India. In clinical studies, availability of small sample size, poor controls, insufficient comparison data between other treatments or placebos or compound, and inconsistent description of treatment/ product are some of the holdings back factors due to which research scenario for herbal entities are struggling. We need to adapt advanced technological interventions right from laboratory to production level in order to overcome such challenges. Government policies are mandatory along with the contribution from pharmaceutical players.
The article is written by Dr. Smarta (CMD-Interlink) & published by Trinity mirror. The link is https://www.trinitymirror.net/news/phytodrugs-new-paradigm-in-healthcare/